Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) accreditation is one of the most consequential compliance hurdles for Medicare suppliers in the United States. For organizations seeking to bill Medicare Part B for DMEPOS items or services, understanding the DMEPOS accreditation process isn’t just bureaucratic, it’s foundational to survival.
In 2026, the stakes are higher than ever: CMS has transitioned to annual accreditation surveys and tightened oversight to improve program integrity, tighten quality, and reduce fraud. In this article, we’ll walk through everything from basic definitions to real-world compliance pitfalls, especially focusing on what practitioners, pharmacists, and supplier managers need to know to plan effectively.
What Is DMEPOS Accreditation?
At its core, DMEPOS accreditation is a certification that a supplier (whether a durable medical equipment provider, prosthetics vendor, orthotics business, or related supplier) meets a comprehensive set of quality standards defined by the Centers for Medicare & Medicaid Services (CMS). These standards cover:
- Administration and financial management
- Human resources and training
- Consumer services and patient follow-up
- Product safety, inventory management, and documentation
- Delivery, installation, and beneficiary education
Obtaining and maintaining accreditation from a CMS-approved accrediting organization is a mandatory condition of Medicare enrollment for most suppliers. Accreditation serves as a quality-control signal to CMS and to beneficiaries that your business is compliant, competent, and legitimate.
💡 Important Clarification: Accreditation is distinct from Medicare enrollment itself. You must be accredited to enroll as a DMEPOS supplier and to retain billing rights; accreditation is not granted automatically with a Medicare provider number.
Why DMEPOS Accreditation Matters in 2026
For decades, accreditation has been a triennial requirement. However, recent regulatory changes (effective January 1, 2026) mandate annual accreditation surveys for all DMEPOS suppliers as a condition of Medicare participation.
Why the shift?
- CMS has identified systemic vulnerabilities across the DMEPOS accreditation program, noting instances where noncompliant suppliers maintained accreditation for years.
- Annual accreditation increases touchpoints between suppliers and accrediting organizations, reducing the time a non-compliant supplier might operate unchecked.
- The change aligns DMEPOS with higher-risk areas of Medicare oversight.
- The new cadence requires suppliers to develop proactive compliance programs and internal audit cycles rather than periodic preparation bursts.
💡Practical impact: Accreditation is now continuous rather than periodic. Suppliers that treat accreditation as a “checkpoint event” risk failing annual surveys and facing Medicare billing termination or corrective action plans.
Federal DMEPOS Accreditation Requirements in 2026
CMS outlines specific requirements for DMEPOS accreditation. While individual accrediting organizations may add interpretive details, the federal baseline includes:
Core Requirements
- Every physical location that supplies DMEPOS must be separately accredited for the product categories it furnishes.
- Suppliers must enroll in Medicare as a DMEPOS supplier and maintain a valid Provider Transaction Access Number (PTAN).
- A surety bond (often required at the time of enrollment) must be posted and kept current.
Quality standards published by CMS must be applied consistently across business operations and documented thoroughly.

Accreditation Organizations
CMS designates and oversees approved accrediting organizations (AOs) that conduct surveys and issue accreditation. These AOs must themselves meet federal standards to be CMS-approved.
Annual Surveys
- Effective 2026, initial accreditation continues to be followed by annual surveys rather than every three years.
- Surveys may include unannounced site visits, records reviews, staff interviews, beneficiary interactions, and inventory inspections.
DMEPOS Accreditation Requirements: Practical Checklist
Before applying for DMEPOS accreditation, or preparing for an annual renewal in 2026, suppliers should confirm that their day-to-day operations truly reflect CMS quality standards, not just on paper but in practice. Surveyors evaluate how policies are implemented, not simply whether they exist.
Administrative Infrastructure
A compliant administrative foundation demonstrates that your organization is stable, accountable, and capable of supporting Medicare beneficiaries long-term.
- Written policies and procedures for all core business functions. Policies should clearly define responsibilities for intake, billing, delivery, complaint handling, returns, and emergency situations. Surveyors often test whether staff can explain these policies in their own words.
- Standard operating procedures for equipment delivery, training, and follow-up. SOPs must outline how equipment is delivered, how beneficiaries are educated on safe use, and how follow-up is conducted to confirm effectiveness, fit, and patient understanding.
Patient Safety and Quality
Patient safety is one of the most heavily scrutinized areas of federal DMEPOS accreditation and a frequent source of deficiencies.
- Training protocols for beneficiaries and caregivers. Documentation should show how and when patients are trained, including written instructions, demonstrations, and confirmation of understanding. Surveyors may request proof that education is tailored to patient needs.
- Documented product safety checks. Suppliers must demonstrate routine inspection, maintenance, and cleaning procedures for equipment. This includes tracking serial numbers, service logs, and manufacturer guidelines.
- Employee competency assessments. Staff involved in patient care, delivery, or setup must be trained and periodically evaluated. Competency should be documented through training records, certifications, and performance reviews.
Records and Documentation
Accurate, consistent documentation is essential for passing both accreditation surveys and Medicare audits.
- Accurate medical and business records. Records should clearly support medical necessity, delivery confirmation, and ongoing use. Discrepancies between clinical documentation and billing data are a common red flag.
- Documentation of quality audits. Internal quality reviews should be performed regularly and documented, including findings, trends, and actions taken to address deficiencies.
- Inventories tagged and matched with billing files. Physical inventory must align with billing records. Surveyors often verify that billed items can be traced to specific inventory records and delivery confirmations.
Compliance Programs
A strong compliance program shows CMS and accrediting bodies that your organization actively monitors risk rather than reacting to problems after they occur.
- Internal quality assurance audits. These audits should review patient files, billing accuracy, delivery documentation, and complaint resolution processes on a scheduled basis.
- Corrective action plans for identified issues. When issues are found, suppliers must document how they were addressed, who was responsible, and how recurrence will be prevented.
- Staff compliance training. Ongoing education ensures employees understand Medicare rules, fraud prevention, documentation standards, and patient rights.
Annual Readiness
With annual accreditation surveys now required, continuous preparedness is critical.
- Mock surveys. Simulated audits help identify weaknesses before an official survey. These should mimic unannounced visits and include staff interviews.
- Peer reviews. Cross-department or peer reviews provide an objective assessment of compliance and operational consistency.
Updates to policy manuals. Policies must be reviewed and updated at least annually to reflect regulatory changes, operational shifts, and lessons learned from audits or complaints.

DMEPOS Accreditation Cost – What to Expect
Unlike a simple one-time fee, DMEPOS accreditation cost varies widely depending on:
- The accrediting organization you choose
- The size and number of locations being accredited
- The breadth of your product categories
- Whether your initial survey finds compliance issues
Typical cost categories include:
| Cost Category | What It Covers | Approximate Range* |
| Application Fee | Submission processing | $500–$2,000 |
| Survey Fee | On-site survey review | $2,000–$7,000+ |
| Documentation Review | Accreditation documentation audit | $1,000–$4,000 |
| Annual Renewal | Ongoing accreditation confirmation | $1,000–$5,000 |
| Corrective Action Support | Assistance with Plan of Correction | Variable |
💡Note: *Costs fluctuate based on accreditor, location, and service scope; always verify with the accrediting organization.
Hidden costs to plan for:
- Staff time for preparation and remediation
- Training and internal audit expenses
- Updating policy and compliance systems
- Potential consultant fees for first-time accrediting
Many suppliers underestimate the indirect resource commitment, not just the monetary cost, required to survive annual accreditation.
Required Postings for DMEPOS Accreditation
CMS and many accrediting organizations require visible postings and consumer materials in your facility, including:
- Supplier Standards poster: must be displayed prominently where patients and caregivers can see it.
- Complaint process information: phone numbers, Medicare contact info, and procedure for submitting complaints.
- Notice of privacy practices: HIPAA and data protection notices.
- Patient education tools: materials explaining rights and how to use equipment safely.
Failure to maintain required postings for DMEPOS accreditation during a site survey is a common deficiency area that results in corrective actions or delays.
DMEPOS Accreditation for Pharmacies
Pharmacies that furnish DMEPOS items (e.g., walkers, glucose monitors, CPAP supplies) face unique compliance intersections between pharmacy law and DMEPOS requirements.
Trade and enrollment guidance confirm: pharmacies must be accredited by a CMS-approved organization to supply DMEPOS products unless they qualify for an exemption.
This DMEPOS accreditation for pharmacies interacts with pharmacy operations in ways that create specific planning and compliance challenges:
- Inventory control must separate DMEPOS from drug stock for documentation and reconciliation.
- Staff must be trained on DMEPOS standards distinct from pharmacy interpersonal skills.
- Billing systems must adequately segregate DMEPOS claims from drug claims for Medicare reporting.
In short, pharmacy DMEPOS compliance is more than a label, it permeates daily operations.
DMEPOS Accreditation Exemption: How It Works
Not all pharmacies must undergo full accreditation. CMS allows a DMEPOS accreditation exemption for pharmacies meeting stringent criteria:
✔ The pharmacy has been enrolled as a DMEPOS supplier for at least 5 years
✔ It has had no unrescinded final adverse actions in the past five years
✔ Medicare billing for DMEPOS items (excluding drugs) is less than 5% of total pharmacy sales for the past three years
Important nuances:
- This is an exemption from the accreditation requirement, not an exemption from all compliance obligations. Pharmacies must still abide by DMEPOS quality standards and are subject to audits.
- If a pharmacy loses the exemption or changes ownership, it must pursue full accreditation.
- The exemption is a strategic compliance decision, not a default path.
Common Pain Points and Compliance Pitfalls
Even experienced suppliers struggle with accreditation – here’s where organizations most often run into trouble:
1. Documentation Gaps
Incomplete or inconsistent record keeping is the #1 deficiency cited in surveys. Review your processes quarterly, not just before accreditation.
2. Policy Overload Without Practice
Large policy manuals that aren’t implemented operationally don’t pass surveys. Focus on practice alignment first, policy second.
3. Failed Mock Surveys
Organizations that don’t simulate unannounced surveys often encounter surprises. Conduct at least one internal mock survey annually.
4. Staff Turnover
Losing trained compliance champions before a scheduled survey is high risk. Always have cross-training.
5. Confusion Between DMEPOS and Pharmacy Compliance
Many pharmacies treat DMEPOS as an add-on. In reality, DMEPOS compliance must be integrated into pharmacy SOPs, quality systems, and training platforms.
How Technology Helps Reduce DMEPOS Accreditation Risk
Many of the most common DMEPOS accreditation failures don’t stem from a lack of intent or effort, they stem from fragmented systems, manual processes, and limited visibility across operations. Documentation gaps, billing errors, inventory mismatches, and inconsistent staff workflows are difficult to control when information lives in multiple platforms or on paper.
This is where purpose-built HME | DME software platforms like NikoHealth become a meaningful compliance advantage rather than just an operational upgrade.
Centralized Documentation That Stands Up to Surveys
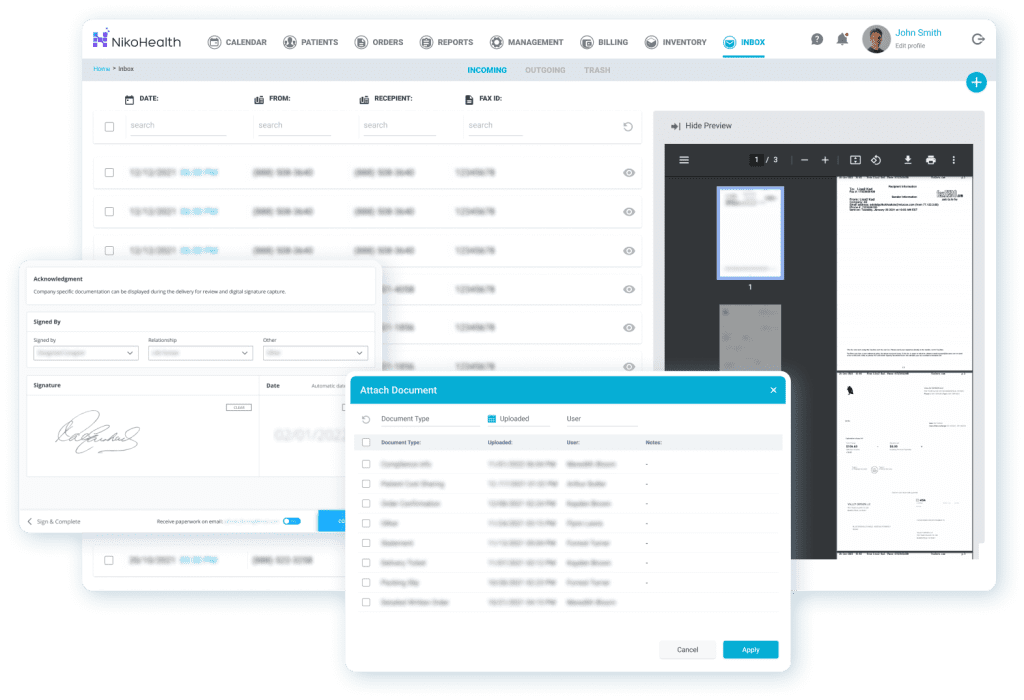
Accreditation surveys live and die by documentation. NikoHealth provides a unified, cloud-based document management system that keeps patient records, delivery confirmations, physician orders, and compliance files connected in one place. This reduces the risk of missing records during surveys and makes it easier to demonstrate consistent compliance across locations and departments.
Billing Accuracy That Supports Compliance
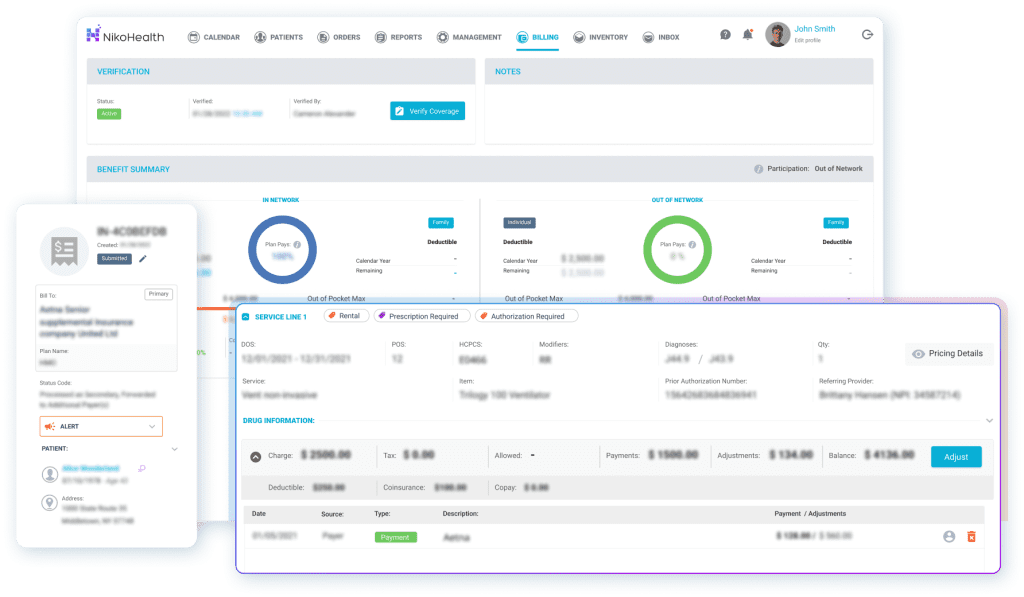
Billing errors are a common trigger for deeper audits and corrective action plans. NikoHealth’s billing and revenue cycle tools are built specifically for HME/DME suppliers, with configurable payer rules, documentation verification, and standardized billing playbooks that help teams submit clean claims the first time. By enforcing compliance checks before submission, suppliers reduce denials, prevent mistakes, and protect revenue within a single system.
By reducing manual billing steps and increasing claim accuracy, suppliers can lower denial rates, shorten time to payment, and reduce compliance risk tied to inconsistent or unsupported claims.
Inventory Control That Matches What’s Billed

Surveyors often verify that billed DMEPOS items can be traced to actual inventory and delivery records. NikoHealth’s real-time inventory management tools help ensure that products are properly tracked from intake through fulfillment, delivery, and billing.
This level of traceability directly supports accreditation requirements related to product safety, delivery verification, and record integrity.
Workflow Consistency Across Teams and Locations
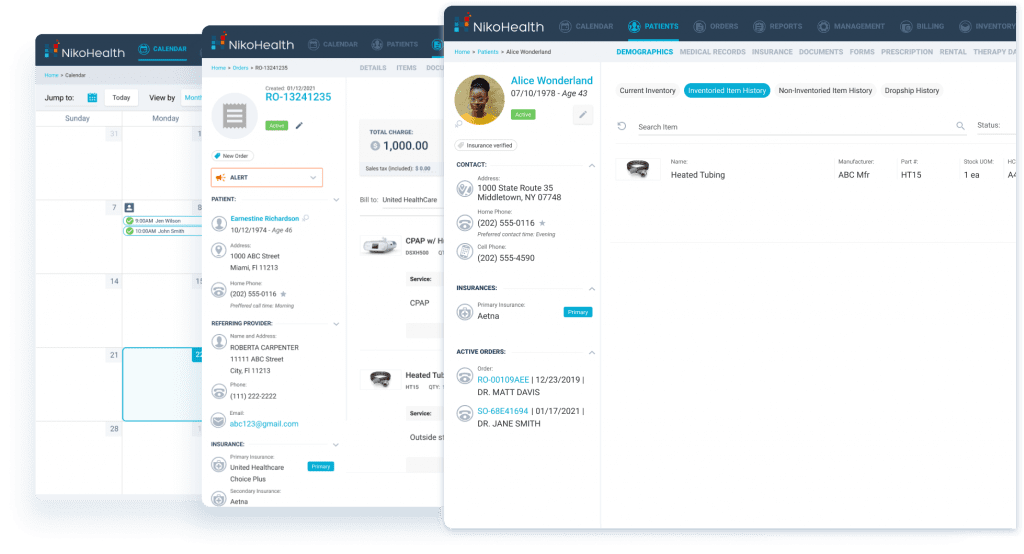
Staff turnover and inconsistent training are recurring compliance pain points. NikoHealth’s intuitive user experience and standardized workflows make it easier to onboard new employees and ensure that processes are followed consistently – whether staff are handling intake, billing, delivery, or resupply.
Built-in reporting and real-time insights also allow leadership teams to monitor performance, identify gaps early, and address issues before they surface during an accreditation survey.
A Platform Built for the Reality of HME/DME Operations
Rather than forcing HME and DME suppliers to adapt generic healthcare software, NikoHealth is designed specifically for the complexities of this industry, including Medicare billing rules, documentation standards, inventory tracking, and audit readiness.
By unifying billing, documentation, inventory, orders, patients, scheduling, reporting, and built-in payer rules and requirements within one ecosystem, NikoHealth enables organizations to shift from reactive compliance to proactive, audit-ready operations.
Conclusion
In 2026, DMEPOS accreditation isn’t a static event, it’s an ongoing commitment to quality, documentation, and patient safety. Suppliers, pharmacies, and stakeholders must elevate their internal compliance programs to survive annual surveys, navigate exemptions responsibly, and remain eligible for Medicare participation.
Approach accreditation with the seriousness of risk management, the discipline of quality improvement, and the foresight of strategic planning. Those who do will not only satisfy CMS requirements, but also strengthen their operations and patient trust.







Related Articles